Congresses are great forgetting into direct communication with physicians, surgeons, nurses, general practitioners, hospital managers, and payers.
Evidence Communication
The average time reading a branded email is 10 seconds. For content aimed to generate interest, you have 5-30 seconds to grab your customers attention.
Want to tell a story? Need to communicate the success of other customers or describe benefits of your product that cannot be studied in a clinical trial? A white paper is likely what you need and the medical writers at Coreva Scientific can help you achieve it.
Internal or external communications often occur during a meeting or conference call. A slide deck supports you but does not distract from you. Coreva Scientific will help you balance the data with the messaging, staying on point, and remaining compliant.
When sales require a more in-depth but still user-friendly approach to communicate your product’s value, consider a sales brochure. All the key data from your dossier presented in easy reading sections.
Do you want to present the evidence for your product in a scientific setting while keeping it short and visually appealing? Appearances at congresses can get the word out there while a full peer-reviewed publication is being prepared. Your presence at a congress can come in different forms: abstract, symposium, poster and presentation.
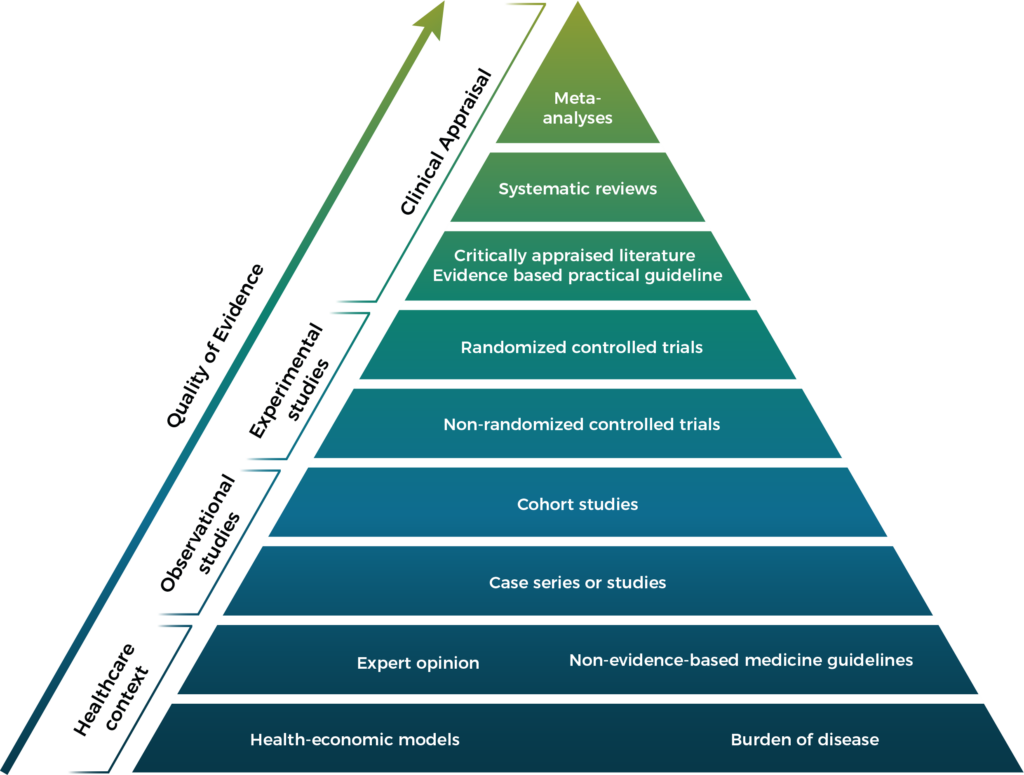
Peer-reviewed publications are the strongest form of evidence. A variety of projects can result in a peer-reviewed publication as illustrated on the pyramid below: