- Aim: To identify, read, and summarize the current literature -> providing you with an overview of the market landscape and any data gaps
- Academic literature databases: PubMed, EMBASE, Cochrane
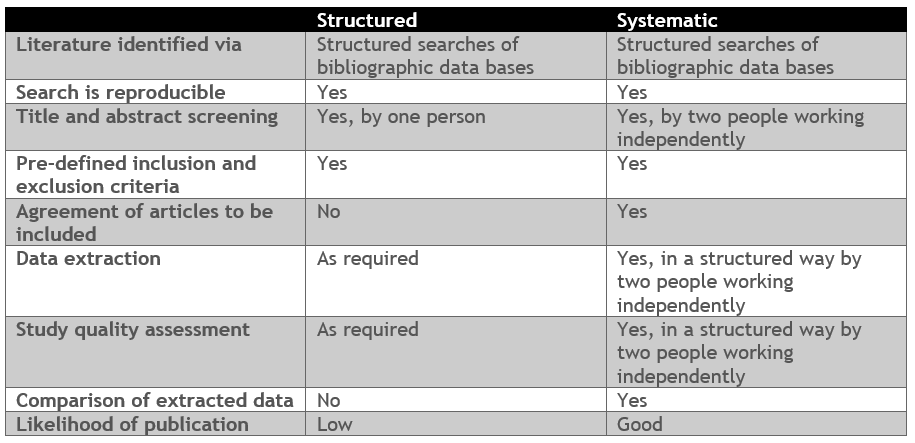
- Systematic or structured reviews
- Regulatory, guideline, and gray literature reviews
- Full PRISMA compliance
- Informs: Decisions on market potential, product development, product acquisition, and future studies
Literature review
A literature review summarizes the information on a topic over a specified time period. The report draws common themes together and provides insight into the longitudinal trends where possible. The key to an informative literature review is identifying (all) the relevant literature. This is done via structured searches of bibliographic databases, making the literature identified transparent and reproducible. A structured search generally returns many more ‘hits’ than can be extensively reviewed, and many may not be pertinent to the research question. For this reason, only a limited set of the hits are read in full and summarized. Those selected for full-text review are identified via screening, in which the title and abstract of each hit is compared against pre-defined inclusion and exclusion criteria.
Unlike a research manuscript, literature reviews does not develop new arguments but considers the arguments of all authors/papers included in the review. They can, though, be published if the review leads to novel insights or identifies important data gaps. For publication, literature reviews in the medical field generally need to be systematic. A systematic review is a type of literature review that collects and critically analyzes multiple research studies and is considered to be one of the highest levels of evidence to inform healthcare decision making. Systematic reviews of randomized controlled trials can be found at the Cochrane library. Coreva Scientific performs both structured and systematic literature reviews, these options are compared below: