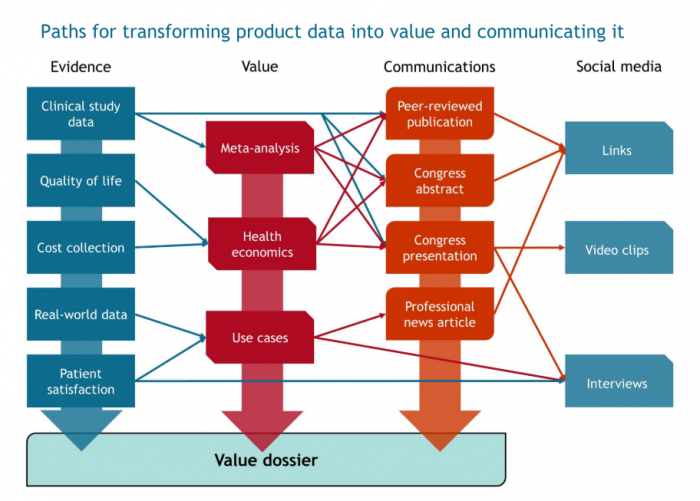
Reimbursement is one gateway to medical device sales. It is not always attainable, and devices often fall under procedure codes. This makes it imperative to have a rounded sales strategy that has a value narrative from concept to long-term value. Here are some key items to keep in mind when building the three pillars of medical device value for your own product.
Clinical value
Efficacy and safety endpoints must be clearly defined and agreed upon by both company and physician stakeholders. Being the best product for a redundant endpoint is of no value. One study may be persuasive, more are better and open the door to meta-analyses and reviews.
Health-economic value
Cost concerns over a product can negate any medical benefit. Health economics combines clinical and cost data to quantify the financial impact of change in healthcare practice. Plan to collect cost and quality-of-life data alongside your clinical studies.
Real-world evidence
Data should also be collected outside of a randomized, controlled trial (RCT). There are concerns that an RCT does not reflect standard practice, making the results non-generalizable. A major oversight is when companies fail to plan for these real-world health outcomes studies.
Burden of disease
Valuable data are of little impact without context. Does your customer understand the unmet needs and cost of treating a particular condition? These details can be crucial to your value messaging hitting its mark.
Value-based healthcare
With cost being a major concern, providers are reluctant to change the status quo and trial a new product. To counter this obstacle, well-designed risk sharing agreements can be implemented, whereby the company is paid only for benefits provided. Options for risk sharing should be considered early, as they can open the door to extensive market penetration.